A research team led by Yuan Zhen, professor in the Faculty of Health Sciences at the University of Macau (UM), has developed a new drug capable of effectively treating meningitis. The research results have been published in the internationally renowned journal Science Advances and have garnered significant attention in the fields of biology and brain science.
In recent decades, the excessive use of antibiotics has led to the emergence and widespread dissemination of multidrug-resistant bacteria, posing a significant challenge to public health. Some of these multidrug-resistant bacteria have even been classified by the World Health Organization (WHO) as ESKAPE pathogens, which require priority attention. One such pathogen is multidrug-resistant Acinetobacter baumannii (A. baumannii). At present, antibiotics available for treating A. baumannii bacterial infections are limited, with options such as tigecycline and colistin, and the emergence of resistant strains to them has been reported. In addition, these antibiotics are associated with significant adverse effects, including nephrotoxicity. Therefore, there is an urgent need to develop new antibiotics to address these issues. Recent studies have focused on the development of metal complexes as novel antibacterial agents against multidrug-resistant bacteria. Metal complexes have a distinct three-dimensional arrangement around one or more metal centres, facilitated by multiple ligands, and exhibit higher bactericidal efficacy, greater targeting specificity, and lower resistance.
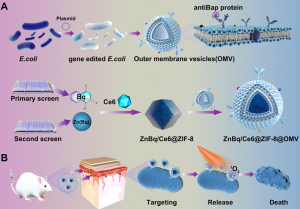
To eradicate multidrug-resistant A. baumannii, the research team carried out a comprehensive screening of over 1,100 Food and Drug Administration-approved small molecule drugs and assessed the efficacy of broxyquinoline (Bq) in combination with various metal ions. Among the compounds examined, the research team identified a metal complex called Zn(Bq)2, which proved to be an exceptional candidate with an ultra-low minimum inhibitory concentration of approximately 0.21μg/mL against A. baumannii.
Effective antibacterial metal complexes often possess hydrophobicity, which might reduce their efficacy. In addition, highly active metal complexes might undergo ligand exchange reactions when exposed to different biological environments. To overcome these limitations and ensure targeted drug delivery and action, the research team has developed a series of innovative approaches. Firstly, outer membrane vesicles (OMVs) with selective targeting capabilities for A. baumannii were genetically engineered. These OMVs can serve as a targeted delivery system to deliver antibacterial metal complexes directly into the target bacteria, thereby enhancing their antibacterial efficacy. Secondly, nano-zeolitic imidazolate framework-8 (ZIF-8) was constructed as a drug carrier. ZIF-8 enables efficient loading and transport of the potent antibacterial metal complex Zn(Bq)2. The use of ZIF-8 enhanced the stability and bioavailability of the metal complex and ensured its efficient delivery to the target bacteria. Using these integrated strategies, the research team achieved a killing efficacy of 99.9999999% against A. baumannii. They also established a mouse model of A. baumannii-induced meningitis and conducted in vivo experiments. The results showed that the engineered nanodrug cured A. baumannii-induced meningitis, highlighting the drug’s potential to treat bacterial infections, especially those that are difficult to eradicate.
This study not only addresses the limitations of traditional antibiotics against A. baumannii, but also provides a targeting strategy using genetically engineered OMVs, representing a significant advance in the field of targeted bacterial eradication. This antibiotic, which can effectively eradicate multidrug-resistant bacteria without causing resistance, also represents a significant advance in the development of non-traditional antibiotics and will help combat the growing global threat of antibiotic resistance.
The corresponding author of this study is Prof Yuan Zhen. His PhD student Wei Xianyuan and former postdoctoral fellow Xue Bin are the co-first authors. Ruan Shuangchen, professor at Shenzhen Polytechnic University, also provided financial support for the study. The research project is funded by the Science and Technology Development Fund of the Macao SAR (File no: 0020/2019/AMJ and 0048/2021/AGJ) and UM (File no: MYRG2020-00067-FHS, MYRG2019-00082-FHS, MYRG2022-00054-FHS, and MYRG-GRG2023-00038-FHS-UMDF). The full version of the research article is available at https://www.science.org/doi/10.1126/sciadv.adk6331.
| Source: Faculty of Health Sciences | |
| Media Contact Information: | |
| Communications Office, University of Macau | |
| Albee Lei | Tel: (853) 8822 8004 |
| Cravina Chong | Tel: (853) 8822 4323 |
| Email: | prs.media@um.edu.mo |