A research team led by Kathy Qian Luo, professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), has unveiled a groundbreaking mechanism driving the malignancy of triple-negative breast cancer (TNBC), opening new avenues for targeted therapies. The study revealed that proprotein convertase subtilisin/kexin type 9 (PCSK9) promotes TNBC progression by depleting cholesterol levels in the plasma membrane, thereby activating oncogenic signalling pathways. These groundbreaking findings suggest that inhibiting PCSK9 or its downstream effectors could significantly suppress TNBC tumour growth and metastasis. The research has been published in the internationally renowned journal Advanced Science.
TNBC, characterised by the absence of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2), is the most aggressive breast cancer subtype with limited treatment options and a high metastatic rate. Although cholesterol metabolism has been associated with cancer progression, the role of PCSK9, a key regulator of cholesterol homeostasis, in TNBC was unclear prior to this study.
The team isolated highly metastatic TNBC cells (4-11) from the lung tumours of mice that had been injected with parental MDA-MB-231 cells. Subsequent RNA sequencing analysis revealed a significant upregulation of PCSK9 in the metastatic cells. The experiment demonstrated that silencing PCSK9 drastically reduced tumour growth and metastasis, while overexpressing PCSK9 enhanced these processes.
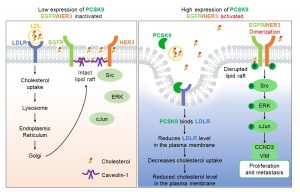
Mechanistically, binding PCSK9 to the low-density lipoprotein receptor (LDLR) reduces cholesterol uptake, leading to cholesterol depletion in the plasma membrane. The depletion of cholesterol disrupts lipid rafts, enabling the activation of epidermal growth factor receptor (EGFR) and HER3. These receptors then drive the Src/ERK/c-Jun signalling cascade, which upregulates cyclin D3 and vimentin to promote cell proliferation and metastasis. The team further validated these findings using inhibitors and activators that target EGFR/HER3 and related downstream kinases. This conclusively demonstrated the pivotal role of PCSK9-driven malignancy. Notably, the artificial depletion of membrane cholesterol or LDLR produced similar pro-metastatic effects, confirming that disruption of lipid rafts is a key trigger for the activation of oncogenic pathways. Analysis of clinical data revealed that TNBC patients with high PCSK9 expression had significantly lower survival rates. By combining cellular biochemistry with clinical evidence, the study offers new strategies for precision treatment of TNBC.
Prof Luo is the corresponding author of the study. FHS PhD graduates Li Tianhong and Wu Renfei are the first and second authors, respectively. The research was funded by the Science and Technology Development Fund (FDCT) of the Macao SAR (File No.: 0147/2020/A3 and 0004/2021/AKP), and the Ministry of Education Frontiers Science Center for Precision Oncology, University of Macau (File No.: SP2021-00001-FSCPO and SP2023-00001-FSCPO). The full version of the research article is available at: https://doi.org/10.1002/advs.202408514.
| Source: Faculty of Health Sciences | |
| Media Contact Information: | |
| Communications Office, University of Macau | |
| Albee Lei | Tel: (853) 8822 8004 |
| Bell Leong | Tel: (853) 8822 8009 |
| Email: | prs.media@um.edu.mo |