A research team led by Zhao Qi, associate professor in the Faculty of Health Sciences at the University of Macau (UM), has made a revolutionary breakthrough in the treatment of bacterial pneumonia. The team has developed pioneering biomimetic nanoantibiotics (BioNanoCFPs) that can penetrate the mucus barrier in the lungs for precise drug delivery to the infection site and enable real-time tracking. This innovation opens new avenues for treating antibiotic-resistant bacterial infections. The research has been published in the prestigious journal ACS Nano and covered by academic media platforms such as iNature and Nano Frontiers.
Pneumonia is one of the top ten causes of death worldwide, claiming over 2.5 million lives each year. Bacterial pneumonia, in particular, poses a significant challenge due to its high complication rate and increasing mortality rate. Although antibiotics remain the primary treatment for bacterial infections, their efficacy is often limited by the complex physiological barriers in the human body. The increased production of mucus in the bronchi and lungs forms a dense layer that obstructs drug penetration and accelerates drug clearance. This leads to an insufficient drug concentration at the infection site, which in turn promotes antibiotic resistance. Only about 2% of traditional intravenous antibiotics can reach the infection site, while the rest are metabolised and lost in the systemic circulation. This not only reduces treatment efficacy but also increases the risk of liver and kidney toxicity. Overcoming the mucus barrier to enable targeted drug delivery is a critical challenge in modern anti-inflammatory therapy.
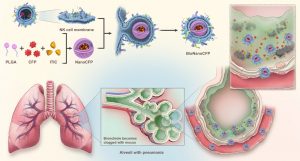
Inspired by the innate immune system, the UM research team focused on the distinctive functions of natural killer (NK) cells. These immune cells are rich in Toll-like receptors (TLRs) on their surface, which enable them to recognise pathogen-associated molecular patterns within bacteria and penetrate biological barriers to reach the infection site. The team pioneered the technology for NK cell membrane-mediated drug delivery, and developed BioNanoCFPs by encapsulating cefoperazone (CFP) and indacenodithieno[3,2-b]thiophene (ITIC) in nanoparticles coated with NK cell membranes. These nanoplatforms have three key functions: (1) Smart navigation: TLRs and adhesion molecules on NK cell membranes are retained to enable the carriers to lock onto infection signals like natural immune cells, penetrate the mucus layer, and reach Klebsiella pneumoniae clusters; (2) Real-time tracking: fluorescence labelling of ITIC facilitates the visualisation of the targeting and treatment processes through imaging, thus optimising dosing; (3) Targeted release: the acidic microenvironment at bacterial colonisation sites triggers the release of CFP from the carriers, which significantly increases the local drug concentration compared to traditional drug delivery methods, achieving a potent antibacterial effect while avoiding systemic toxicity.
In mouse models of severe pneumonia, a single dose of BioNanoCFPs demonstrated remarkable efficacy, significantly reducing the bacterial load in the lungs compared to traditional treatments and showing no signs of recurrence. No damage to major organs was observed, and blood biochemical indicators remained normal, confirming the low toxicity of the treatment. The breakthrough lies in its combination of biomimetic and smart functions. It not only retains the antigens and surface structures of the original cells but also endows the nanoplatforms with several biological functions, including specific ligand recognition, targeted disease treatment, and the ability to penetrate biological barriers. With demonstrated efficacy against bacterial pneumonia, BioNanoCFPs hold enormous potential for future clinical applications.
The corresponding authors of the study are Prof Zhao; Gong Ping and Zhang Pengfei, researchers at the Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences; and Cheng Zhen, researcher at the Shanghai Institute of Materia Medica, Chinese Academy of Sciences. The first author is Wang Yue, a doctoral graduate of the Faculty of Health Sciences at UM. The research was funded by the Science and Technology Development Fund of the Macao SAR (File No.: 0009/2023/RIC and 0043/2021/A1), and UM (File No.: MYRG2022-00143-FHS and MYRG-GRG2023-00158-FHS-UMDF). The full version of the research article is available at: https://pubs.acs.org/doi/full/10.1021/acsnano.4c10837.
| Source: Faculty of Health Sciences | |
| Media Contact Information: | |
| Communications Office, University of Macau | |
| Albee Lei | Tel: (853) 8822 8004 |
| Bell Leong | Tel: (853) 8822 8009 |
| Email: | prs.media@um.edu.mo |