A research team led by Kwok Hang Fai, professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), has made significant progress in developing targeted therapies for glioblastoma (GBM), a highly aggressive brain cancer. By combining a novel scorpion venom-derived decapeptide (Ctri9495, C9) with a specifically modified dendritic nanocarrier (PAMAM dendrimer), the team has constructed an innovative and efficient nanoparticle drug delivery system, and named it G5C9. The G5C9 system is not only capable of precisely identifying and efficiently penetrating tumour cells but also can significantly inhibit tumour growth by activating autophagic cell death pathways. This research provides a promising strategy for addressing drug delivery challenges in GBM treatment, holding substantial potential for clinical translation. The findings have been published in the internationally renowned journal Journal of Controlled Release.
GBM is the most common and aggressive primary malignant tumour of the central nervous system, characterised by an extremely poor patient prognosis and a five-year survival rate of only about 5%. Existing chemotherapy drugs, such as temozolomide, often struggle to effectively reach the tumour site due to barriers such as the blood-brain barrier and the blood-brain tumour barrier, while the tumours themselves readily develop drug resistance. Therefore, there is an urgent need for novel therapies that can efficiently penetrate these barriers, precisely target the tumour, and effectively kill cancer cells.
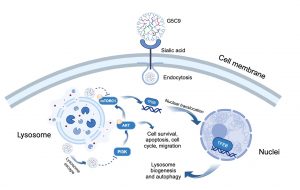
Animal venom peptides, known for their structural diversity and high bioactivity, offer a valuable resource for developing new anti-cancer drugs. However, natural peptide therapeutics often encounter challenges such as insufficient targeting, poor stability in vivo, and difficulties in cell penetration. To address these issues, Prof Kwok and his research team adopted an innovative approach. First, they identified a novel hydrophobic decapeptide, C9, in the venom of the scorpion Chaerilus tricostatus. Then, to enhance C9’s ability to penetrate cells, the team selected a fifth-generation polyamidoamine dendrimer (G5 PAMAM) as the carrier. To add tumour-targeting ability, the team modified the carrier’s surface with 4-(bromomethyl) phenylboronic acid (PBA), which specifically detects and binds to sialic acid that is overexpressed on GBM cell surfaces, acting like an ‘intelligent navigation system’. Finally, the C9 peptide was chemically conjugated to the modified dendrimer to form the G5C9 complex.
The G5C9 complex achieves efficient targeting through the specific binding of PBA to sialic acid on the tumour cell surface. Unlike the free C9 peptide, which only binds to the cell membrane, G5C9 is rapidly endocytosed by GBM cells and primarily localises within lysosomes. G5C9 demonstrates potent anti-tumour activity both in vitro and in vivo, while free C9 peptide shows no significant effect. Importantly, the study has elucidated G5C9’s unique mechanism of action: it downregulates the phosphorylation levels of key proteins in the crucial pro-survival and proliferation PI3K/AKT/mTOR signalling pathway. Furthermore, G5C9 disrupts lysosomal function and inhibits the activity of mTOR complex 1 (mTORC1) located on the lysosomal membrane. This disruption leads to the dephosphorylation and nuclear translocation of Transcription Factor EB (TFEB), the master regulator of lysosomal biogenesis and autophagy. TFEB’s nuclear translocation activates the autophagosome-lysosome pathway (ALP), as evidenced by increased autophagosome formation, elevated levels of the autophagy marker LC3-II, and degradation of the autophagy substrate p62, ultimately resulting in autophagic cell death in GBM cells. This strategy not only significantly enhances the anti-tumour efficacy of the scorpion venom peptide C9 but also provides a new technological platform and theoretical foundation for utilising other bioactive peptides that have potential activity but face delivery challenges in treating brain diseases and other malignancies.
The corresponding author of the study is Prof Kwok. The first author is Qin Haixin, a former postdoctoral fellow in FHS. Significant contributions were also made by Luo Siyuan, a doctoral student in FHS; Zuo Weimin, a doctoral graduate from FHS; Cao Zhijian, professor at Hubei University of Technology; and Yehuda G. Assaraf, professor at the Technion-Israel Institute of Technology and the recipient of 2024 UM Distinguished Visiting Scholar in FHS. The study received crucial support from the Biological Imaging and Stem Cell Core, as well as the Proteomics, Metabolomics and Drug Development Core in FHS. The research project was funded by the Science and Technology Development Fund of the Macao SAR (File No.: 0010/2021/AFJ and 0027/2022/A1), the UM–Dr. Stanley Ho Medical Development Foundation ‘Set Sail for New Horizons, Create the Future’ Grant 2024 (File No.: SHMDF-VSEP/2024/002), and the Ministry of Education Frontiers Science Center for Precision Oncology, University of Macau (File No.: SP2025-00001-FSCPO). The full version of the research article is available at: https://doi.org/10.1016/j.jconrel.2025.113780.
| Source: Faculty of Health Sciences | |
| Media Contact Information: | |
| Communications Office, University of Macau | |
| Albee Lei | Tel: (853) 8822 8004 |
| Bell Leong | Tel: (853) 8822 8009 |
| Email: | prs.media@um.edu.mo |