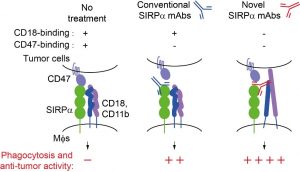
While antibodies that block the inhibitory checkpoints PD-1 or CTLA-4 have achieved remarkable success in recent years, many patients still experience limited or short-lived responses. These challenges have prompted researchers to explore other immune cells, particularly macrophages, which play a key role in tumour immunity. SIRPα is a classical inhibitory receptor on macrophages. When bound to CD47, which is frequently overexpressed on tumour cells, SIRPα sends inhibitory signals that block macrophage-mediated phagocytosis and anti-tumour immunity. Although therapies targeting the SIRPα–CD47 axis can enhance anti-tumour immunity, clinical results have been inconsistent, and some patients have experienced side effects, suggesting that other suppressive mechanisms may be involved.
The UM research team has identified a pathway that helps explain this phenomenon. Their study showed that even when the SIRPα–CD47 interaction was disrupted, macrophages still displayed partial inhibition. They found that SIRPα directly cis interacts with CD18 on the cell surface of macrophages, restraining CD18 activation and macrophage phagocytosis. This SIRPα-CD18 interaction occurs on the same cell surface and works together with the known pathway to suppress the immune response. To translate these findings into potential therapy, the team engineered a bispecific antibody capable of simultaneously blocking both the SIRPα–CD18 and SIRPα–CD47 interactions. This antibody markedly enhanced macrophage phagocytosis and significantly inhibited tumour growth in mouse models, demonstrating stronger anti-tumour effects than antibodies blocking either pathway alone.
Tang notes that immune checkpoints are more complex than initially expected. He emphasises that deeper knowledge of their molecular mechanisms is essential for designing safer and more effective cancer therapies. The team’s study reveals a dual-inhibitory mode of immune regulation and offers valuable insight for optimising next-generation immunotherapies.
The study is co-authored by Tang and André Veillette, professor at the Montreal Clinical Research Institute, with Tang as the first author, and Tang and Veillette as corresponding authors. The research was supported by the Science and Technology Development Fund of the Macao SAR (File No: 0090/2024/RIB2) and UM (File No: SRG2024-00027-FHS and UMDF-TISF/2025/002/FHS). The full version of the study is available at: https://www.science.org/doi/10.1126/sciimmunol.adv5085.
| Source: Faculty of Health Sciences | |
| Media Contact Information: | |
| Communications Office, University of Macau | |
| Albee Lei | Tel: (853) 8822 8004 |
| Bell Leong | Tel: (853) 8822 8009 |
| Email: | prs.media@um.edu.mo |