Every day, often hidden from notice, our bodies are locked in a constant tug-of-war between ‘firefighters’ and ‘fires’. The human immune system, acting as a well-trained fire brigade, springs into action the moment it detects abnormal fire hazards in the body. However, when the immune system becomes overactive, it can spark uncontrolled fires, leading to chronic inflammation. From skin redness and itching to joint pain and even cancer recurrence, these inflammation-related problems can spread like wildfire, threatening our health. At the University of Macau (UM), research teams have taken on the role of ‘immune firefighters’, working to find innovative ways to extinguish these fires. Unlike traditional treatments that only manage inflammation symptoms or come with unwanted side effects, the work of these researchers focuses on addressing the root causes of inflammation, providing better protection for human health.
Rescue for Uncontrolled Skin Inflammation
Skin inflammation is an increasingly common health concern in urban areas. According to statistics from Zhiyan Consulting Group in China, nearly one in ten people in the country suffer from dermatitis, with over 70 million affected by eczema. Many patients experience such intense itching that it disrupts their sleep, and some even scratch their skin until it bleeds, leading to infections and a loss of self-esteem. Traditional steroid treatments can come with serious side effects. While biologics are effective, they are expensive, with annual treatment costs reaching RMB 10,000, making them unaffordable for many families.
Thanks to groundbreaking research at UM, a breakthrough may be on the horizon. Prof Leung Chung Hang, professor in the Institute of Chinese Medical Sciences (ICMS), and his research team have discovered a key mechanism behind skin inflammation—a ‘protein switch’ called Keap1-Nrf2. The Nrf2 protein plays a crucial role in directing cells to fight inflammation and maintain skin homeostasis. However, during inflammation, the Keap1 protein ‘kidnaps’ Nrf2, shutting it down and allowing the inflammation to escalate, similar to a fire that grows more intense over time.
Previous attempts to treat inflammation focused on directly activating Nrf2. However, this approach was much like a random guess at a password, risking interference with other physiological functions and causing unwanted side effects. Prof Leung’s team has made a breakthrough with their ‘protein detective’ technology. By using iridium probes, they are able to tag the Keap1 protein, making it impossible for the protein to hide. Then, with high-throughput screening, they can quickly identify active compounds capable of effectively separating Keap1 from Nrf2, allowing the ‘fire brigade’ to get back to work.
Through systematic screening, the team has also discovered that an extract from a Chinese herbal medicine can precisely dismantle the Keap1-Nrf2 protein complex. This extract not only shows impressive anti-inflammatory effects, but is also safe, gentle, and non-irritating. Based on current cost evaluations, products made with this extract could be widely available at an affordable price.
‘Rather than brutally suppressing symptoms, our technology provides a protective shield for the skin and helps it learn to self-regulate,’ Prof Leung explains vividly. The team is preparing to apply for a patent for their technology and has drawn interest from cosmetics companies, with the first anti-inflammatory skincare product expected to launch in the near future.
Putting Out the ‘Slow-Burning Fire’ of Chronic Inflammation
Chronic inflammation, like a slow-burning fire, can quietly harm the body and last for months or even years. For example, office workers often suffer from chronic muscle strain caused by sitting for long periods and poor posture, which leads to stiff necks and shoulders. Middle-aged and elderly people commonly experience osteoarthritis or arthritis, while sports enthusiasts may develop chronic tendinitis from ligament and tendon injuries caused by overtraining or insufficient recovery.
To address these issues, Prof He Chengwei, associate professor in ICMS, working with his team, systematically screened dozens of medicinal plants. They discovered that Glycine tabacina (煙豆), a plant commonly found in Fujian and Taiwan, possesses remarkable anti-inflammatory potential. In traditional Chinese medicine, Glycine tabacina is used to relieve dampness, support the spleen and kidneys, and strengthen muscles and bones. However, its anti-inflammatory efficacy and mechanisms have not been fully explored. Through modern pharmacological research, Prof He’s team revealed for the first time that coumestrol and isoflavonoids in Glycine tabacina can precisely regulate multiple inflammatory signalling pathways, including SYK, TAK1, PI3K, and NF-κB. Functioning like a ‘precision firefighting system’, these compounds not only reduce chronic inflammation but also promote bone repair and improve osteoporosis.
Building on this discovery, the team combined traditional wisdom with modern technology to develop ‘Macao One Root’—a topical adhesive that can relax muscles, improve circulation, and effectively alleviate chronic inflammatory pain. This innovative product has already secured four invention patents, and the team is collaborating with medical product companies to scale up production. Prof He explains that the Chinese nickname for Glycine tabacina, ‘One Root’ (一條根), comes from a folk tale about a legendary herbal remedy in the Ming-Qing Dynasty. According to the tale, residents of the Kinmen Islands brewed tea or wine from Glycine tabacina roots to ease the rheumatic pain of soldiers struggling to adapt to the islands’ climate. ‘With modern technology, we are able to validate this ancestral knowledge. The “Macao One Root” patches honour history while demonstrating the dedication of Macao researchers,’ says Prof He.
Groundbreaking Smart Anti-Inflammatory Biomaterial
In medicine, the balance of macrophages functions like a precise regulator of health. M1 macrophages act as the body’s ‘defence forces’, responsible for attacking pathogens. However, if overactivated, they can cause chronic inflammation and disrupt the wound-healing process. On the other hand, M2 macrophages function as the body’s ‘repair engineers’, promoting tissue regeneration. Yet, an excessive presence of M2 macrophages may promote tumour recurrence or suppress hair follicle regeneration.
To address these challenges, Prof Qu Songnan, professor in the Institute of Applied Physics and Materials Engineering, and his team conducted in-depth analysis and identified three key limitations in current therapies. First, although natural biomaterials like hydrogels can modulate immune responses between M1 and M2 macrophages, the rigid crosslinked structure of hydrogels cannot adapt to the dynamic microenvironment needed for tissue repair. Second, in tumour immunotherapy, tumour-associated macrophages (TAMs) form an immunosuppressive barrier that significantly reduces the effectiveness of treatments. Finally, and most critically, existing treatments for chronic wounds lack precise methods to regulate the immune microenvironment. These findings prompted the team to raise an ambitious question: Could an intelligent material be developed to actively ‘communicate’ with the immune system and tackle these challenges at their root?
After years of research, the team achieved a major breakthrough. By combining natural egg white protein with carbon nanodots, they created an innovative biomaterial called the ‘carbon-dot-linked egg white hydrogel’. This material features three transformative capabilities. First, it has an adaptive dynamic crosslinking structure that responds to the changing needs of tissue regeneration at different stages. Second, it incorporates a chronologically programmed drug delivery system that aligns precisely with therapeutic windows. Finally, and most critically, it can directly reprogramme the immune microenvironment by precisely modulating macrophage phenotypes (M1/M2 polarisation). This breakthrough marks a paradigm shift in biomaterials, evolving them from passive structural carriers into active systems that can modulate immune responses.
When applied to tumour resection sites, the carbon nanodots within the hydrogel function as a sophisticated precision guidance system. They effectively repolarise tumour-associated macrophages, shifting them from pro-tumour M2 phenotypes to tumour-suppressing M1 phenotypes. At the same time, they activate T cell-mediated immunity by disrupting PD-L1 expression on cancer cells. This innovative therapy has demonstrated remarkable efficacy in animal trials, significantly reducing tumour recurrence rates and presenting a groundbreaking postoperative treatment strategy.
The smart hydrogel also offers a revolutionary solution for chronic wounds. By releasing stage-specific regenerative signalling molecules, it transforms overactive M1 macrophages into pro-healing M2 phenotypes, significantly reducing the risk of amputations in diabetic patients. Equally impressive is its efficacy in hair follicle regeneration. The hydrogel’s degradation rate naturally synchronises with the hair growth cycle, while its precise immunomodulation activates dormant follicular stem cells, providing new hope for patients suffering from alopecia.
Prof Qu and his team’s groundbreaking research has been published in leading international journals, including Advanced Science and Advanced Materials, and has been granted invention patents in mainland China. The project is now incubated at the UM Centre for Innovation and Entrepreneurship, with clinical trials expected to begin in two years. Emphasising the broader impact of their work, Prof Qu says, ‘This is not just a new material. It also serves as a versatile therapeutic platform with the potential to transform treatments for immune-related diseases.’
UM’s Anti-Inflammatory Philosophy
From persistent skin rashes and chronic joint pain to cancer recurrence, many seemingly unrelated health issues stem from a common root cause: immune system dysregulation. Instead of relying on conventional immunosuppressive approaches, UM’s ‘fire brigade’ is pioneering innovative methods to reboot the immune system and restore the body’s natural balance. We look forward to seeing these groundbreaking studies move from the laboratory to clinical applications and, ultimately, to the market. This will offer hope to countless individuals in overcoming the challenges of inflammation and reclaiming their health and well-being.
Text: Kelvin U
Photo: Provided by the interviewees
English Translation: Kelvin U, Bess Che
Source: UMagazine ISSUE 31

Prof Leung Chung Hang
流程-1-300x173.jpg)
Competitive luminescence-based high-content screening (HCS) process for identifying anti-inflammatory compounds

Prof He Chengwei
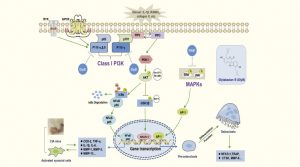
The molecular mechanisms underlying the anti-inflammatory properties of Glycine tabacina

Prof Qu Songnan
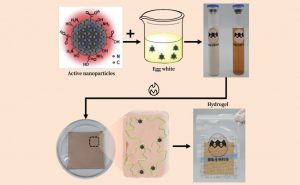
Synthesis of the ‘carbon-dot linked egg white hydrogel’

