Lei Chon Lok, assistant professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), in collaboration with research teams from the University of Nottingham and Cornell University, has developed a new computational model for patch-clamp experiments. This model enhances understanding of how electrical signals work in cells, such as nerve and heart muscle cells, by revealing previously undetectable details of how they function. The new technique opens up new avenues for electrophysiology research and provides fresh insights into disease diagnosis and drug safety testing. The research has been published in the top international journal Advanced Science.
Electrophysiology is the study of how cells, especially nerve and heart muscle cells, use electrical signals to function and communicate. These signals are vital to the human body, underpinning thought, movement, and the heartbeat. In other words, they are the ‘electrical language’ of life. One of the most important tools in this field is the patch-clamp experiment, which enables researchers to measure tiny electrical changes in individual cells with remarkable precision. This experiment measures and controls the membrane potential of a cell while simultaneously measuring the ionic currents flowing across the cell membrane, which is crucial for understanding how cells generate electrical impulses.
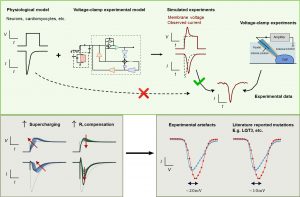
Although the patch clamp experiment has long been a cornerstone of cellular electrophysiology, scientists have often struggled to accurately measure and interpret electrical signals recorded from cells. Many of these recordings, including information crucial for safety considerations, can be misinterpreted due to subtle experimental distortions, also known as artefacts. To address this challenge, the research team has developed an innovative computational model that can effectively identify and correct these artefacts in patch-clamp experiments. The model simulates the influence of experimental tools and conditions, including amplifier settings and cell properties, on the recorded data. It integrates various technical parameters, such as capacitance, resistance, and voltage offset, and has been tested in a series of experiments involving different cell types and setups, including human stem cell-derived heart muscle cells and genetically modified kidney cells.
When the team applied the model to the cardiac fast sodium current, a key factor in heart muscle function, they successfully resolved experimental artefacts by linking observed current recordings with simulated membrane voltage. This explains the shifts and delays in recorded currents that have previously puzzled researchers. The study also showed that typical current-voltage data artefacts can explain biases that had previously been attributed to disease-related genetic mutations or drug effects, highlighting the importance of using the new computational model for accurate data interpretation.
Improving the precision of these patch-clamp recordings enables the computational model to reveal the true electrical behaviour of cells, providing access to important information that was previously obscured by technical limitations. This enables electrophysiological research to make fitting and recover precise estimates of ionic current properties under a wider range of experimental conditions. This breakthrough not only enhances the reliability of related research but also contributes to a deeper understanding of heart rhythm disorders, neurological disorders, and other diseases related to cellular electrical activity, as well as drug effects.
Prof Lei is the co-first and corresponding author of the study. Alexander Clark, a former researcher at Cornell University, contributed to the experimental work, and Gary Mirams, professor at the University of Nottingham, assisted in the design of the computational model. Other team members also provided support at different stages of the research. The project was funded by the Science and Technology Development Fund of the Macao SAR (File No.: 0155/2023/RIA3 and 0048/2022/A), and the University of Macau (File No.: SRG2024-00014-FHS). The full version of the research article is available at: https://advanced.onlinelibrary.wiley.com/doi/full/10.1002/advs.202500691.
| Source: Faculty of Health Sciences | |
| Media Contact Information: | |
| Communications Office, University of Macau | |
| Albee Lei | Tel: (853) 8822 8004 |
| Bell Leong | Tel: (853) 8822 8009 |
| Email: | prs.media@um.edu.mo |